
This is because the heat is coming from decaying fuel, not an active fission reaction. With all reactors shut down, if fighting or other problems cause another loss of external power, plant operators would have more time to arrange a backup power source to run the cooling systems, and the cooling load would be decreased, Arndt said. WHAT HAPPENS IF EXTERNAL POWER IS LOST AGAIN? Still, International Atomic Energy Agency Director-General Rafael Grossi said Sunday he remains “gravely concerned about the situation at the plant, which remains in danger as long as any shelling continues.” Oak Ridge National Laboratory, said in an interview Sunday. “A cold shutdown enormously reduces the meltdown risk,” Steven Arndt, president of the American Nuclear Society and a scientist at the U.S. This is a nuclear power plant’s safest operating mode. With all nuclear reactions stopped, temperatures and pressure inside reactors gradually decline, reducing the required intensity of water cooling of the radioactive fuel. When external power was restored using a reserve transmission line, they executed a “cold shutdown” of the sixth reactor - inserting control rods into the reactor core to stop the nuclear fission reaction and generation of heat and pressure. They needed power from at least one reactor to run the safety systems.

But when all external power was lost, they couldn’t shut down all the reactors.

The Zaporizhzhia plant’s Ukrainian operators apparently decided that it was too risky to operate any of the six reactors, because the fighting had endangered external power supplies for cooling and other safety systems. Here is a look at the risks, impact and what could be done if external power is lost again. On Sunday, one plant connection to Ukraine’s power grid was restored, so the sixth reactor’s power wasn’t needed for the safety systems. This “island mode” is unreliable and not designed to be more than a stopgap measure, Ukrainian officials say. 5 fire caused by shelling knocked the plant off of all external transmission lines, the sixth reactor had had to keep operating - at reduced output - to power reactor cooling and other crucial safety equipment. That power is needed to prevent the reactors from overheating to the point of a meltdown that could breach the surrounding concrete and steel containment buildings and spew radiation through Ukraine, Russia and other nearby countries.

The last of the Russian-occupied Zaporizhzhia plant’s six nuclear reactors was shut down Sunday because Russia’s military actions in Ukraine had repeatedly cut reliable external power supplies. Fe or Au: the atomic radius of iron is 156 pm while that of gold is 174 pm.KYIV, Ukraine (AP) - The forced shutdown of Ukraine’s endangered and crippled Zaporizhzhia nuclear power plant - Europe’s largest - significantly reduces the risk of a radiation disaster that has haunted the world for weeks. Si or S: the atomic radius of silicon is 111 pm while that of sulphur is 88 pm.Ĩ. Be or Ba: the atomic radius of berryllium is 112 pm while that of barium is 253 pm.ħ. Cl or Br: the atomic radius of chlorine is 79 pm while that of bromine is 94 pm.Ħ. O or C: the atomic radius of oxygen is 48 pm while that of carbon is 67 pm.ĥ. Ga or B: the atomic radius of gallium is 136 pm while that of boron is 87 pm.Ĥ. Ca or Ni: the atomic radius of calcium is 194 pm while that of nickel is 149 pm.ģ.
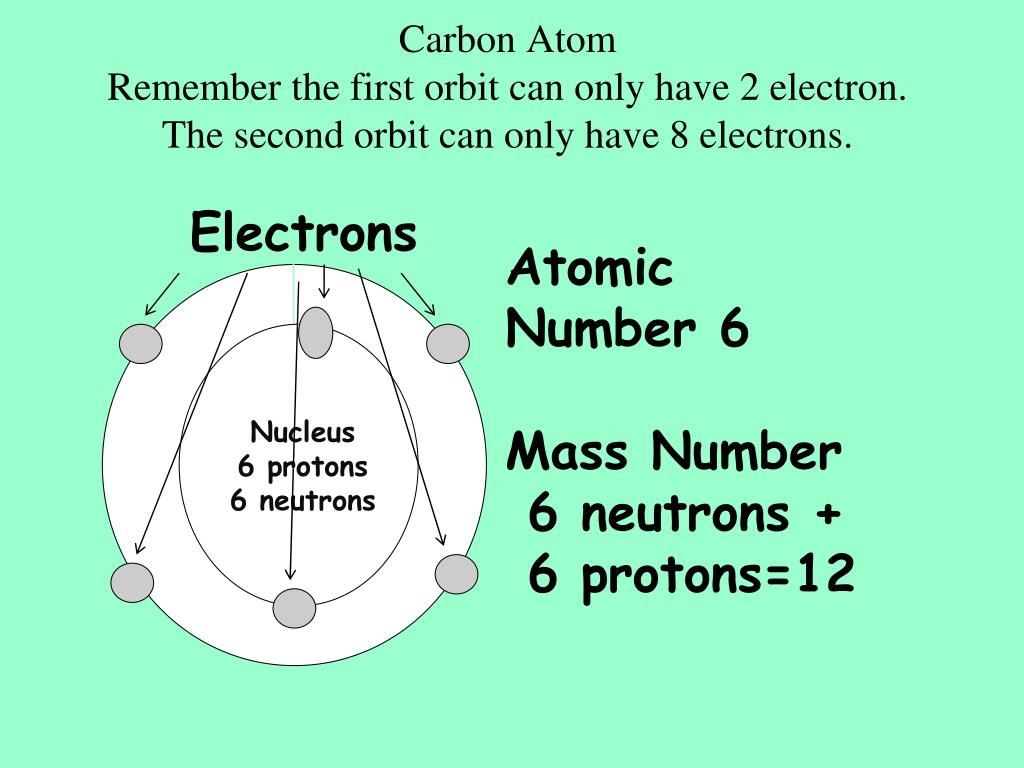
Li or K: the atomic radius of lithium is 167 pm while that of potassium is 243 pm.Ģ. Also, the atomic radius of a chemical element increases down each group of the periodic table, typically from top to bottom (column).Īdditionally, the unit of measurement of the atomic radius of chemical elements is picometers (1 pm = 10 - 12 m).ġ. The atomic radius of a chemical element decreases across the periodic table, typically from alkali metals (group one elements such as hydrogen, lithium and sodium) to noble gases (group eight elements such as argon, helium and neon). Atomic radius can be defined as a measure of the size (distance) of the atom of a chemical element such as hydrogen, oxygen, carbon, nitrogen etc, typically from the nucleus to the valence electrons.


 0 kommentar(er)
0 kommentar(er)
